Chemistry Regents Which of the Following Blue Aqueous
Weight measure of matter and Earths gravitational pull. These short objective type questions with answers are very important for Board exams as well as competitive exams like UPSC NDA SSC etc.

E3 Chemistry Guided Study Book 2020 School Edition High School Chemistry With Nys Regents Exams The Physical Setting Eyo Effiong 9781086782844 Amazon Com Books
Tartrate ions 3S 2 O 8 2 2H 2 O 2CO 2 2HCO 2 6H 6SO 4 2 OH OH CO 2 O 2 C.
. An aqueous solution turns red litmus solution blue. The middle element selenium has an atomic mass that is close to the sum of the atomic masses of sulfur and tellurium divided by 2. The salt NaC2H3O2 can be derived from the reaction of a Aacidic and turns litmus blue Bacidic and turns litmus red Cbasic and turns litmus blue.
Has luster b Malleable c Ductile d Brittle e Good conductor 5. Excess addition of which of the following solution would reverse the change. Carbohydrates give Molischs test.
C ethanol and methanoic. 3 An aqueous solution of compound X reacts with aqueous sodium hydroxide to form a green precipitate and then aluminium powder is added. 47 When the pH of an aqueous solution is changed from 1 to 2 the concentration of hydronium ions in the solution is 1 decreased by a factor of 2 2 decreased by a factor of 10 3 increased by a factor of 2 4 increased by a factor or 10.
The overall equation for this reaction is shown. None of the above statements a-c are correct e. Based on this result the solution is 1 organic 3 basic 2 inorganic 4 acidic 37 Given the reaction.
Free download in PDF Organic Chemistry Multiple Choice Questions and Answers for competitive exams. 1 Na2O 2 K2O. Chemistry Regents Exam Created Date.
A ammonium nitrate B copperII chloride C ironII nitrate D ironIII chloride. To answer this you must know which groups are the following reagents used to identify. D 2When aqueous solutions of S 2 O 8 and tartrate ions are mixed the reaction proceeds very slowly.
Solutions are homogeneous mixtures. 6 50704 Nov02 5 A student prepared ethene from a hydrocarbon oil using the apparatus shown below. It can be prepared by the reaction between.
1 6 An ester has the structural formula shown below. Ablue to green b green to blue c orange to green d green to orange if this indicator is used when titrating an unknown strong base by adding a strong acid the color of the indicator will change from a basic due to the production of h3oions b acidic due to the production of h3oions cbasic due to the production of ohions d acidic due to. Which of the following is NOT a property of a metal.
HSO 4 Ôø½ HPO 4 2Ôø½ SO 4 2Ôø½ H 2 PO 4 Ôø½ Which pair represents an acid and its conjugate base. Aqueous solution of which of the following oxides can turn blue litmus red. Each pH unit change is.
These short solved questions or quizzes are provided by Gkseriesp. Mus paper turns blue. Solutions can have color.
Matter its characteristics Matter- anything that has mass and takes up space. For exam- ple solutions of copper salts have a characteristic blue color while a solution of sodium nitrate is col- orless. In a neutral solution H OH- d.
However this reaction proceeds quickly in the presence of an Fe3aq catalyst. L bromcresol green 2 bromthymol blue 3 litmus 4 methyl orange Based on the data table which unknown solution could be 01 M NaOH. Solutions will pass through a filter.
Up to 24 cash back Chapter 1 Intro to Chemistry Chemistry Matter Chemistry the study of matter and its changes. 1 298 K 3 27C. 3 P2O5 4MGO.
Triad 1 shown in the table below consists of sulfur selenium and tellurium. Tollens test is given by aldehydes and alkynes. In a basic solution OH- H c.
What type of element is it. COO hydrogen carbonate HCO 3 chlorate ClO 3 halides Cl Br I when combined with Ag Pb2 or Hg 2 2 sulfates SO 4 2 when combined with Ag Ca2 Sr2 Ba2 or Pb2 Ions That Form InsolubleCompounds Exceptions carbonate CO 3 2 when combined with Group 1 ions or ammonium NH 4 chromate CrO. A sample of an element is shiny is a good electrical conductor and does not shatter when hit with a hammer.
Mass measurement showing the amount of matter. A methanol and methanoic acid. Conducts electricity turns blue litmus to red.
Dobereiner noticed a relationship between the atomic masses of the elements in each triad. The mixture is heated and a gas that turns damp red litmus paper blue is given off. Up to 24 cash back Aqueous Dissolved in water aq o NaCl aq NaCl NaCl solute Water solvent Maer-Mixtures-Homogeneous-Solution-aq-Solvent- Solute-Heterogeneous-PureSubstances-Elements-PeriodicTable-Cantbe broken down-1 symbol CorCa-Compounds-Canbe brokendown chemically-2ormore symbols NaCl.
Firstly let us draw the structures of benzoic acid and phenol. In an acidic solution H OH- b. You can use this to find out the appropriate test to distinguish between phenol and benzoic acid.
41 Which temperature represents the highest average kinetic energy of the particles in a sample of matter. Regents Chemistry Exam Explanations January 2017. Regents review ABS Astrong acid with a weak base Bstrong acid with a strong base Cweak acid with a weak base Dweak acid with a strong base 33An aqueous solution of NaC2H3O2 is basic.
Thus hexan-1-ol can be converted into hexylamine by the following two reactions I II CH3CH25OH CH3CH25Cl CH3CH25NH2 hexan-1-ol 1-chlorohexane hexylamine whereas neither of the following two reactions takes place. 2 267 K 4 12C. A Baking powder b Lime c Ammonium hydroxide solution d Hydrochloric acid - Get the answer to this question and access a vast question bank that is tailored for students.
The reaction is an example of a cracking b oxidation c polymerisation d saturation. B methanol and ethanoic acid. Solutions are clear and do not disperse light.
All of the above statements a-c are correct. 1 HSO 4 Ôø½and SO 4 2Ôø½ 3 SO 4 2Ôø½and H 2 PO 4 Ôø½ 2 HSO 4 Ôø½and HPO 4 2Ôø½ 4 SO 4 2Ôø½and HPO 4 2Ôø½. Circle one METALNONMETALMETALLOID 6.
A student tested a 01 M aqueous solution and made the following observations. Up to 24 cash back to blue as the pH of the solution is changed from 55 to 80. Solutions will not settle on standing.
Which of the following statements isare correct.

Colour Ions In Aqueous Solution Chemistry Lessons Organic Chemistry Books Chemistry Practical

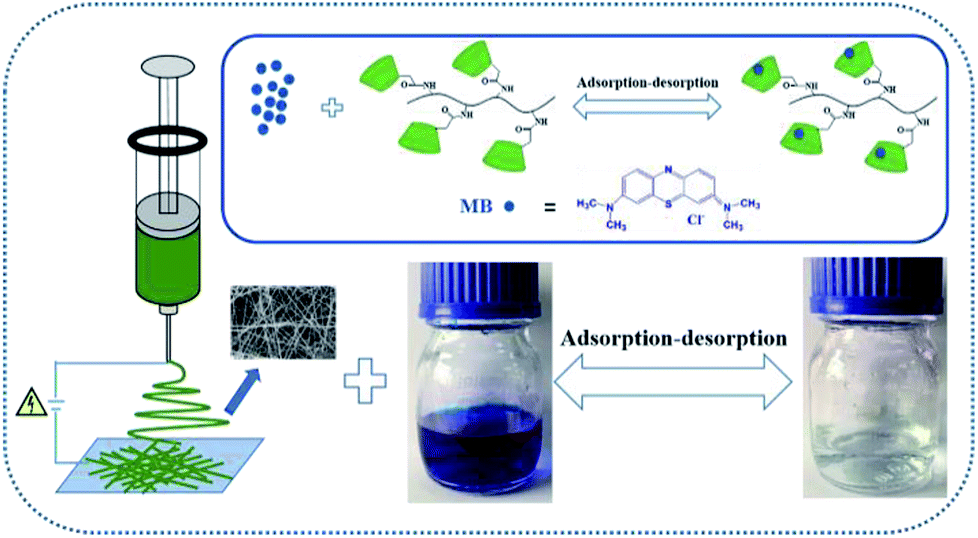
Fabrication Of Eco Friendly Nanofibrous Membranes Functionalized With Carboxymethyl B Cyclodextrin For Efficient Removal Of Methylene Blue With Good Recyclability Rsc Advances Rsc Publishing

Surviving Chemistry 3 Book Bundle 2015 Revisions A Whole Year Of Hs Chem Book Bundles Chemistry Teaching Chemistry
No comments for "Chemistry Regents Which of the Following Blue Aqueous"
Post a Comment